As you may be aware, on April 19th CMS released the FY 2020 SNF PPS Proposed Rule that sets forth the proposed updates to FY 2020 beginning Oct 1, 2019 . As you know, CMS finalized the Patient Driven Payment Model in last year’s final rule, so the proposed rule contains mostly expected revisions related to the new payment model.
However, CMS also uses more than half (147 pages) of the 232-page document to detail significant proposed updates to the IMPACT act quality reporting program (QRP). It is important that providers understand the proposed updates to the PDPM as well as the future of QRP. These have been detailed these here.
FY 2020 SNF PPS/PDPM Updates
-
CMS has proposed a Market Basket Update of 2.5%. This equates to $887 million in aggregate payments to SNFs during FY 2020.
-
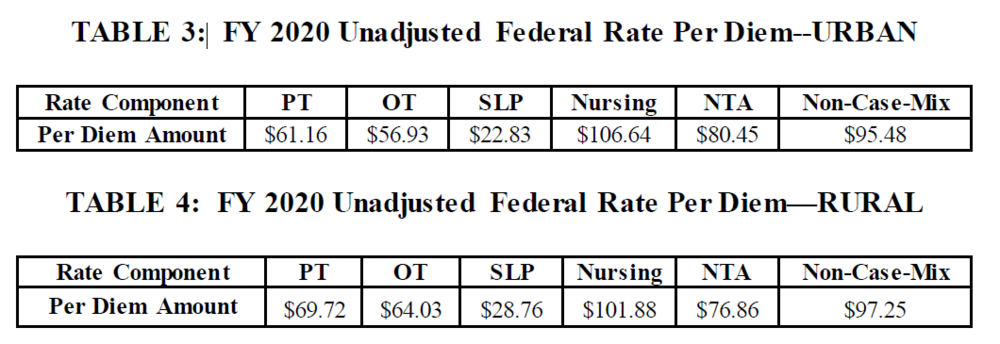
Base Rates for all PDPM Payment categories have all been updated:
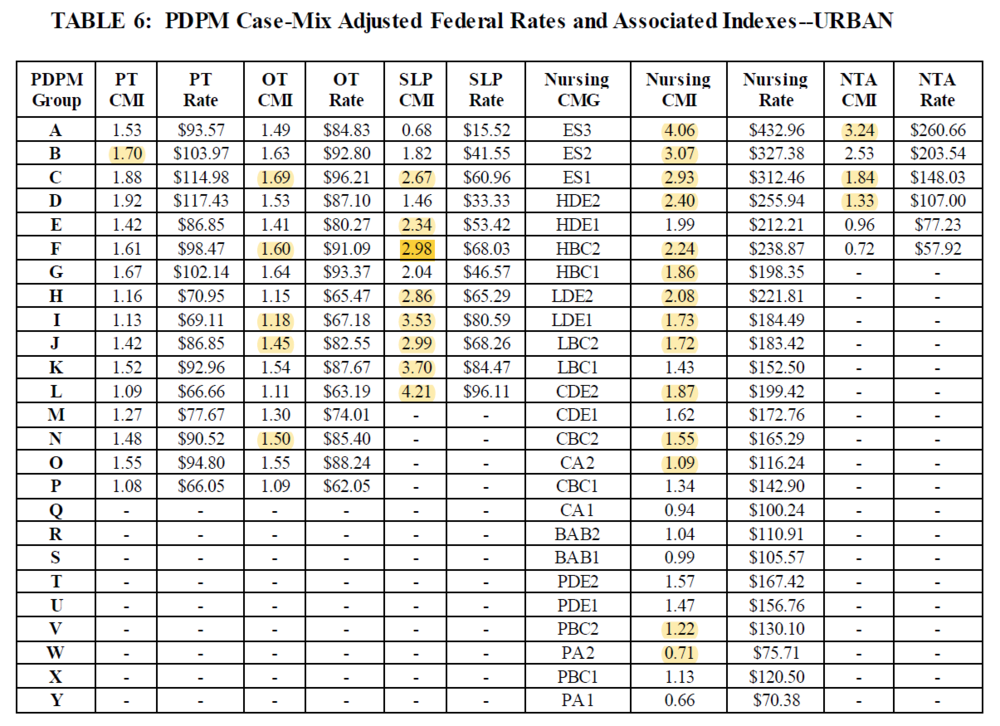
3. Several CMIs have been revised (see highlighted revised CMIs)
4. The Relative Importance Factor has been updated.
a. Labor Related: 0.708
b. Non-Labor Related: 0.292
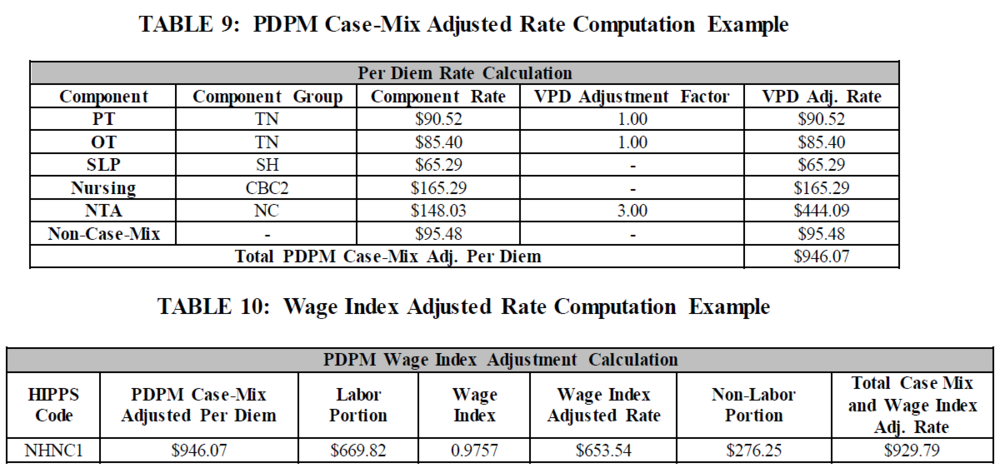
5. Wage Index Adjusted Rate Calculation same as FY 2019:
The total case-mix adjusted per diem rate is the sum of all five case-mix adjusted components into which a patient classifies, and the non-case-mix component rate.
In order to calculate the labor portion of the case-mix adjusted per diem rate, one would multiply the total case-mix adjusted per diem rate by the FY 2020 labor-related share percentage. The remaining portion of the rate would be the non-labor portion.
The final case mix adjusted rate would be the sum of the Wage index adjusted labor related portion of the total case-mix adjusted per diem rate and the non-labor related portion of the total case-mix adjusted per diem rate.
Example (using Wage Index 0.9757):
6. Updated Wage Indexes: can be found here.
SNF-Level of Care – Administrative Presumption
CMS is retaining the Administrative Level of Care Presumption defined at section 30.1 of CMS Pub. 100-2 Chap.8 with modifications to accommodate the differences between RUG IV and the PDPM. CMS continues to believe that this designation reflects an administrative presumption that those beneficiaries who are correctly assigned one of the designated case-mix classifiers on the 5-day Medicare-required assessment are automatically classified as meeting the SNF level of care definition up to and including the assessment reference date (ARD) for that assessment. This presumption recognizes the strong likelihood that those beneficiaries who are assigned one of the designated case-mix classifiers during the immediate post-hospital period would require a covered level of care, which would be less likely for other beneficiaries.
Group Therapy Redefined
CMS is proposing to define group therapy in the SNF Part A setting as a qualified rehabilitation therapist or therapy assistant treating two to six patients at the same time who are performing the same or similar activities. CMS believes this definition would offer therapists more clinical flexibility when determining the appropriate number for a group, without compromising the therapist’s ability to manage the group and the patient’s ability to interact effectively and benefit from group therapy. CMS also believes this revised definition would support CMS’ cross-setting initiatives under the IMPACT Act and Meaningful Measures Initiative, and would align the definition of group therapy used under the SNF PPS more closely with the definitions used within the outpatient setting covered under Medicare Part B and under the IRF PPS, and that this type of standardization would reduce administrative burden on providers by utilizing the same or similar definitions across settings.
Sub Regulatory Process for Updating ICD-10 Initiated
CMS indicates that it is essential that they are able to update the code mappings and lists consistent with the latest coding guidance. Therefore, to ensure that the ICD-10 mappings and lists used under PDPM reflect the most up to date codes possible, CMS is proposing to update any ICD-10 code mappings and lists used under PDPM, as well as the SNF GROUPER software and other such products related to patient classification and billing, through a subregulatory process which would consist of posting updated code mappings and lists on the PDPM website.
Beginning with the updates for FY 2020 , nonsubstantive changes (changes limited to those specific changes that are necessary to maintain consistency with the most current ICD–10 medical code data set) to the ICD-10 codes included on the code mappings and lists under the PDPM would be applied through this subregulatory process. Substantive revisions (changes that go beyond the intention of maintaining consistency with the most current ICD-10 medical code data set. For instance, changes to the assignment of a code to a comorbidity list or other changes that amount to changes in policy) to the ICD–10 codes on the code mappings and lists used under the PDPM would be proposed and finalized through notice and comment rulemaking.
Quality Reporting Program (QRP) Updates
1. CMS is proposing to expand data collection for the SNF QRP quality measures to all SNF residents, regardless of payer source.
2. Current SNF QRP Measures
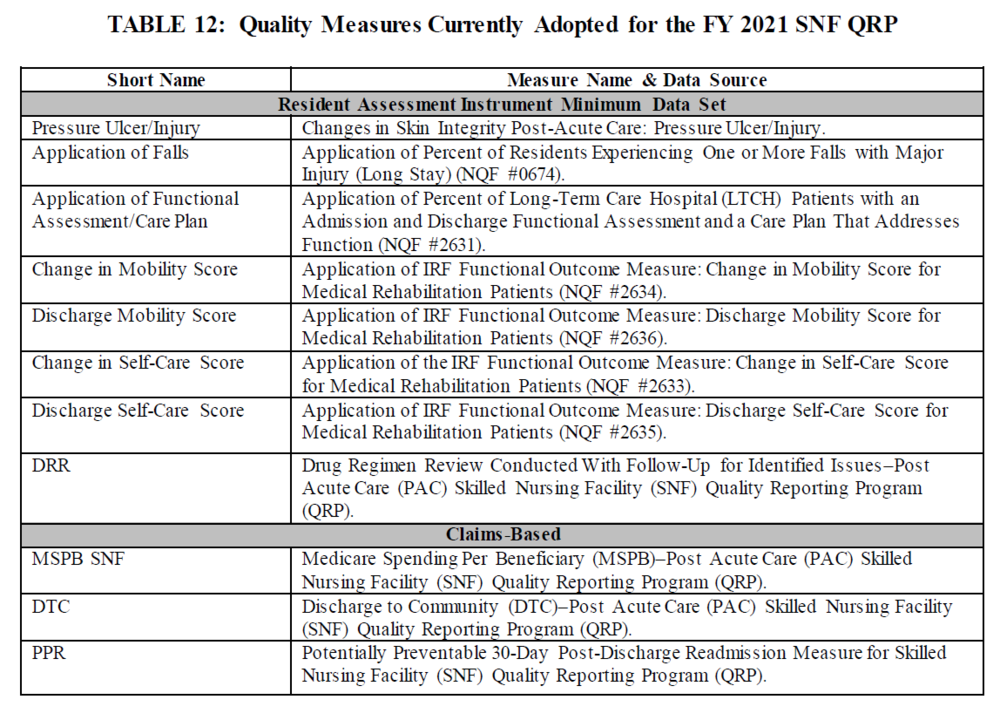
3. 2 New Proposed QRP Measures to begin to be reported FY 2022 (Both of these proposed measures support CMS’s Meaningful Measures priority of promoting effective communication and coordination of care, specifically the Meaningful Measure area of the transfer of health information and interoperability):
► (1) Transfer of Health Information to the Provider–Post-Acute Care (PAC); assesses for the timely transfer of health information, specifically a reconciled medication list. This measure evaluates for the transfer of information when a patient is transferred or discharged from their current setting to a subsequent provider defined as a short-term general hospital, a SNF, intermediate care, home under care of an organized home health service organization or hospice, hospice in an institutional facility, an IRF, an LTCH, a Medicaid nursing facility, an inpatient psychiatric facility, or a critical access hospital.
SNF Denominator
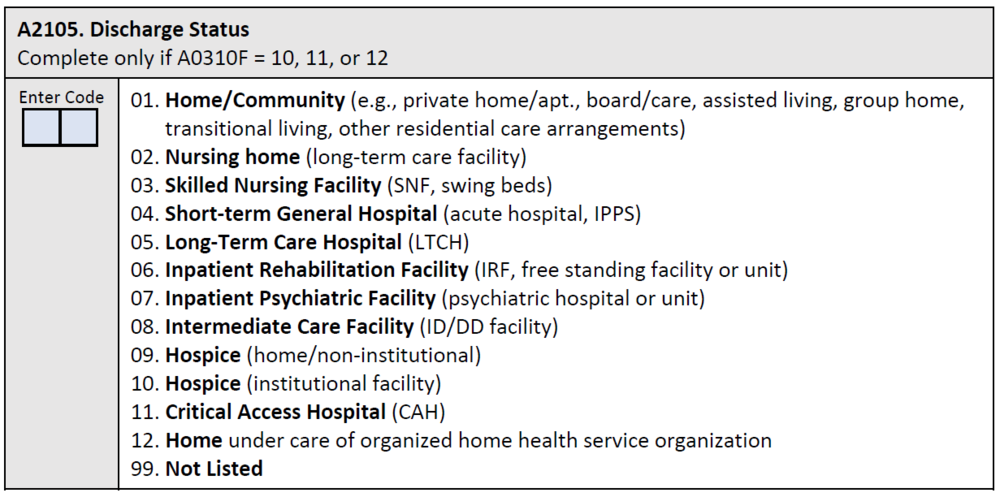
The denominator is the total number of SNF Medicare Part A covered resident stays ending in discharge to a short-term general hospital, another SNF, intermediate care, home under care of an organized home health service organization or hospice, hospice in an institutional facility, a swing bed, an IRF, an LTCH, a Medicaid nursing facility, an inpatient psychiatric facility, or a critical access hospital. Discharge to one of these providers is determined based on response to the discharge location item, A2105, of the MDS assessment, shown below. A stay is defined as the time period from resident admission or reentry to the facility (identified by a 5-day PPS assessment) to discharge.
SNF Numerator
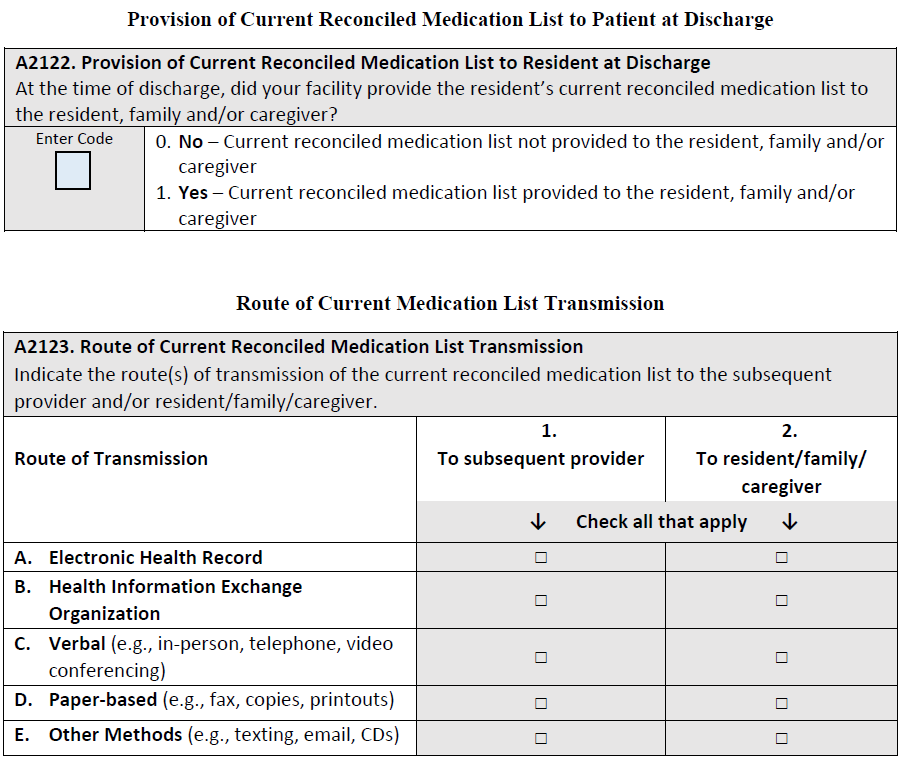
The numerator is the number of stays for which the MDS 3.0 indicated that the following is true: At the time of discharge, the facility provided a current reconciled medication list to the subsequent provider (A2121= [1]).
Items Included in the Quality Measure
One data element will be included to calculate the measure. One data element will be collected to inform the internally consistency logic of the proposed measure
► (2) Transfer of Health Information to the Patient–Post-Acute Care (PAC). This proposed measure assesses for and reports on the timely transfer of health information, i.e., a current reconciled medication list, to the patient/resident when discharged from their current setting of post-acute care to a private home/apartment, board and care home, assisted living, group home, transitional living, or home under the care of an organized home health service organization or hospice.
SNF Denominator
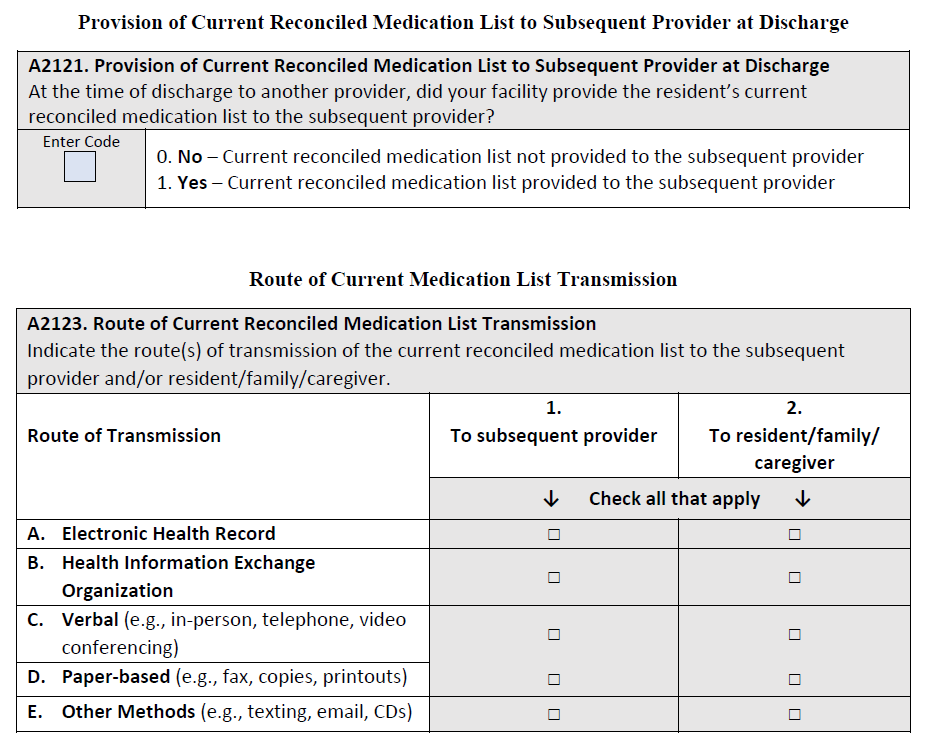
The denominator for this measure is the total number of SNF Medicare Part A covered resident stays ending in discharge to a private home/ apartment (apt.), board/care, assisted living, group home, transitional living or home under care of organized home health service organization or hospice. Discharge to one of these locations is determined based on response to the discharge location item, A2105, of the MDS assessment, shown below. A stay is defined as the time period from resident admission or reentry to the facility (identified by a 5-day PPS assessment) to discharge.

SNF Numerator
The numerator is the number of stays for which the MDS 3.0 indicated that the following is true: At the time of discharge, the facility provided a current reconciled medication list to the resident, family and/or caregiver (A2122= [1]).
4. CMS is proposing to update the specifications for the Discharge to Community–PAC SNF QRP measure to exclude baseline nursing facility (NF) residents from the measure. Baseline residents are residents who lived in a NF prior to their SNF stay and may not be expected to return to the community following their SNF stay.
5. Standardized Patient Assessment Data Elements (SPADEs): The Improving Medicare Post-Acute Care Transformation Act of 2014 (IMPACT Act) requires CMS to develop, implement, and maintain standardized patient assessment data elements (SPADEs) for post-acute care (PAC) settings. The four PAC settings specified in the IMPACT Act are home health agencies (HHAs), inpatient rehabilitation facilities (IRFs), long term care hospitals (LTCHs), and skilled nursing facilities (SNFs). The goals of implementing cross-setting SPADEs are to facilitate care coordination, interoperability, and improve Medicare beneficiary outcomes.
Existing PAC assessment instruments (i.e., OASIS for HHAs, IRF-PAI for IRFs, LCDS for LTCHs, and the MDS for SNFs) often collect data elements pertaining to similar concepts, but the individual data elements — questions and response options — vary by assessment instrument. With a few exceptions, the data elements collected in these assessment instruments are not currently standardized or interoperable, therefore, patient responses across the assessment instruments cannot be compared easily.
The IMPACT Act further requires that the assessment instruments described above be modified to include core data elements on health assessment categories and that such data be standardized and interoperable. Implementation of a core set of standardized assessment items across PAC settings has important implications for Medicare beneficiaries, families, providers, and policymakers. CMS is proposing standardized patient assessment data elements for five categories specified in the IMPACT Act. These categories are:
-
Cognitive function (e.g., able to express ideas and to understand normal speech) and mental status (e.g., depression and dementia)
-
Special services, treatments, and interventions (e.g., need for ventilator, dialysis, chemotherapy, and total parenteral nutrition)
-
Medical conditions and co-morbidities (e.g., diabetes, heart failure, and pressure ulcers)
-
Impairments (e.g., incontinence; impaired ability to hear, see, or swallow)
-
Other categories as deemed necessary by the Secretary
CMS has finalized the adoption of SPADEs for two of the categories (1) Functional status: Data elements currently reported by NFs to calculate the measure Application of Percent of Long-Term Care Hospital Patients with an Admission and Discharge Functional Assessment and a Care Plan That Addresses Function (NQF #2631); and (2) Medical conditions and comorbidities: the data elements used to calculate the pressure ulcer measures, Percent of Residents or Patients with Pressure Ulcers That Are New or Worsened (Short Stay) (NQF #0678) and the replacement measure, Changes in Skin Integrity Post-Acute Care: Pressure Ulcer/Injury.
CMS is also proposing that SNFs would be required to report an extensive new group of SPADEs beginning with the FY 2022 SNF QRP. If finalized as proposed, SNFs would be required to report these data with respect to SNF admissions and discharges that occur between October 1, 2020 and December 31, 2020 for the FY 2022 SNF QRP. Beginning with the FY 2023 SNF QRP, CMS proposes that SNFs must report data with respect to admissions and discharges that occur during the subsequent calendar year (for example, CY 2021 for the FY 2023 SNF QRP, CY 2022 for the FY 2024 SNF QRP). The following is a list of the proposed SPADEs. This document offers an much more thorough explanation of the proposed SPADEs listed below as well as examples of the proposed data elements as they would appear in assessment tools, most of which have been modified from the way they appear in the current assessment tools, including the MDS. On a recent Open-Door Forum, CMS indicated that these additional proposed SPADEs, while not part of any formal QRP measure, would be subject to the QRP APU requirements.
A. SPADEs for Cognitive function (e.g., able to express ideas and to understand normal speech) and mental status (e.g., depression and dementia)
1. The Brief Interview for Mental Status (BIMS)
2. The Confusion Assessment Method (CAM)
3. Mental Status (Depressed Mood) PHQ-2 to 9
B. SPADEs to Assess for Special Services, Treatments, and Interventions
1. Chemotherapy
2. Radiation
3. Oxygen Therapy
4. Suctioning
5. Tracheostomy Care
6. Non-invasive Mechanical Ventilation
7. Invasive Mechanical ventilation
8. IV Medications (Antibiotics, Anticoagulation, Vasoactive Medications, Other)
9. Transfusions
10. Dialysis (Hemodialysis, Peritoneal dialysis)
11. V Access (Peripheral IV, Midline, Central line)
12. Parenteral/IV Feeding
13. Feeding Tube
14. Mechanically Altered Diet
15. Therapeutic Diet
16. High-Risk Drug Classes: Use and Indication (anticoagulants; antiplatelets; hypoglycemics (including insulin); opioids; antipsychotics; and antibiotics)
C. SPADEs to Assess for Medical Conditions and Co-Morbidities
1. Pain Interference
D. SPADEs to assess for Impairments
1. Hearing and Vision Impairments
2. Vision
E. SPADEs to assess for a new category: Social Determinants of Health
1. Race and Ethnicity
2. Preferred Language and Interpreter Services
3. Health Literacy
4. Transportation
5. Social Isolation
6. CMS also posted concepts of Proposed future QRP measures and SPADES that are under consideration.
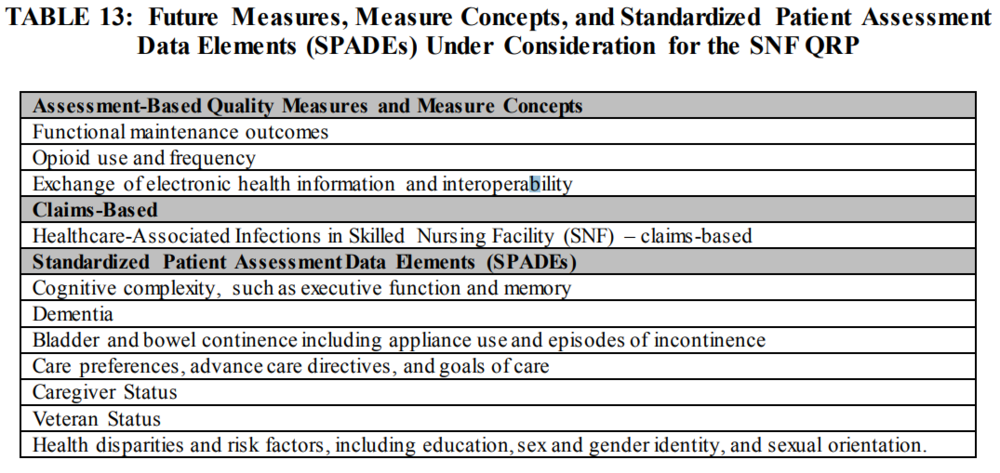
7. SNFs are currently required to submit MDS data to CMS using the Quality Improvement and Evaluation System (QIES) Assessment and Submission Processing (ASAP) system. CMS will be migrating to a new internet Quality Improvement and Evaluation System (iQIES) that will enable real-time upgrades over the next few years, and CMS is proposing to designate that system as the data submission system for the SNF QRP once it becomes available, but no later than October 1, 2021. CMS is proposing to replace the Survey Provider Enhanced Reports (CASPER)” with “CMS designated data submission”. CMS is also proposing to replace the reference to the “Quality Improvement Evaluation System (QIES) Assessment Submission and Processing (ASAP)” with “CMS designated data submission” and replace the reference to “QIES ASAP” with “CMS designated data submission system” effective October 1, 2019. In addition, CMS is proposing to notify the public of any future changes to the CMS designated system using subregulatory mechanisms, such as website postings, listserv messaging, and webinars.
8. CMS is proposing to begin publicly displaying data for the Drug Regimen Review Conducted With Follow-Up for Identified Issues-Post Acute Care (PAC) Skilled Nursing Facility (SNF) Quality Reporting Program (QRP) measure beginning CY 2020 or as soon as technically feasible.
Proposed SNF Value Based Purchasing Updates
1. The SNFPPR and the SNF QRP potentially preventable readmission measures assess different aspects of SNF care, CNS has received stakeholder feedback that having two SNF potentially preventable readmission measures has caused confusion. To minimize the confusion surrounding these two different measures, CMS is changing the name of the SNFPPR to Skilled Nursing Facility Potentially Preventable Readmissions after Hospital Discharge.
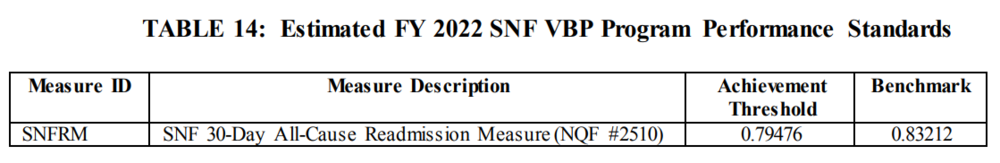
2. FY 2022 Performance Period and Baseline Period for Subsequent Years
A. The performance period for the FY 2022 program year will be FY2020, and the baseline period will be FY 2018.
B. CMS is proposing that SNFs would have 30 days from the date that they issue VBP reports to review the claims and measure rate information and to submit to us a correction request if the SNF believes that any of that information is inaccurate. CMS indicates that this 30-day review and correction period would commence on the day that they issue the June report, and a SNF would not be able to request that CMS correct any underlying claims or its measure rate after the conclusion of that 30-day period. This proposal would change the deadline from March 31st of the following year.
B. SNF VBP Impact to SNFs for FY 2020
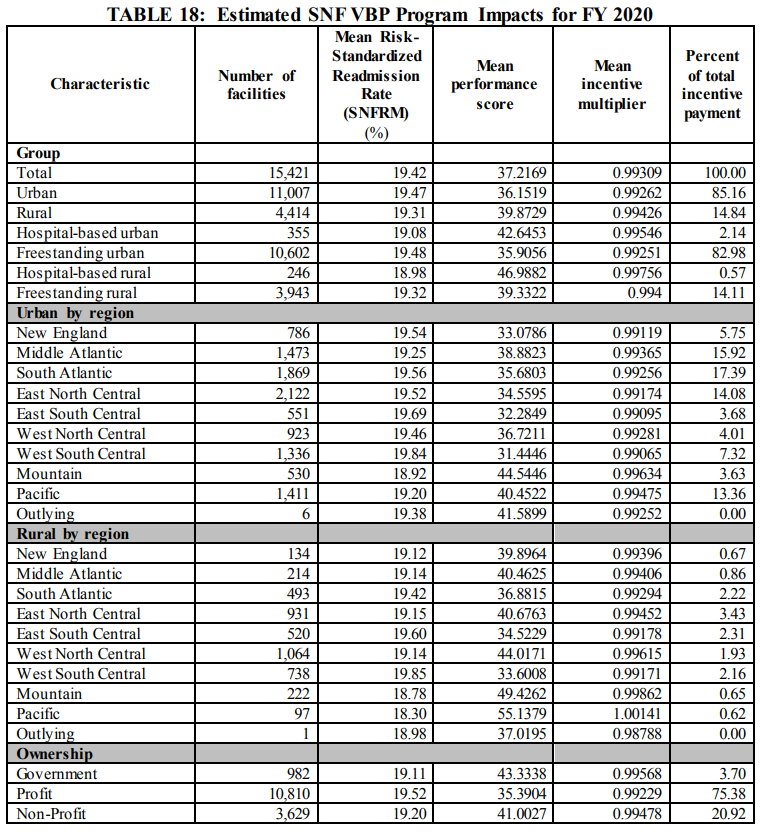
FY 2020 Proposed Rule Impact Analysis
A. Information Collection Requirements
1. CMS estimates that the total number of PPS 5-day assessments, PPS discharge assessments, and IPAs that would be completed across all facilities will be 4,905,042 assessments (2,406,401 + 2,406,401 + 92,240, respectively). The total estimated time for all assessments across all facilities is 4,169,286 hours per year (4,905,042 assessments x 0.85 hours/assessment). For all assessments across all facilities, CMS estimates a burden of $280,421,251 (4,905,042 assessments x $57.17/assessment).
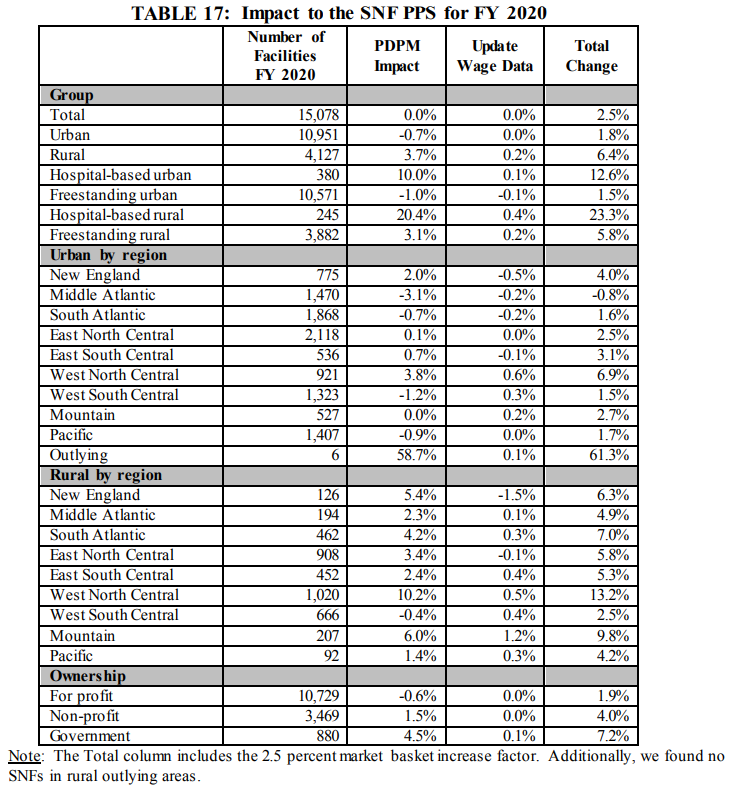
2. Overall Impact to SNFs